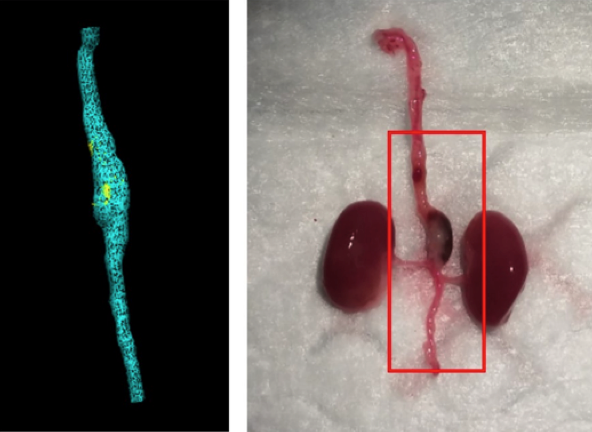
Creating aneurysms in mice permits us to examine the way(s) in which macrophage functionality impacts aneurysm. At left, an in vivo, 3-dimensional, ultrasound image of an AngiotensinII-induced aneurysm in a mouse. At right, the ex vivo imaging of the same aneurysm.
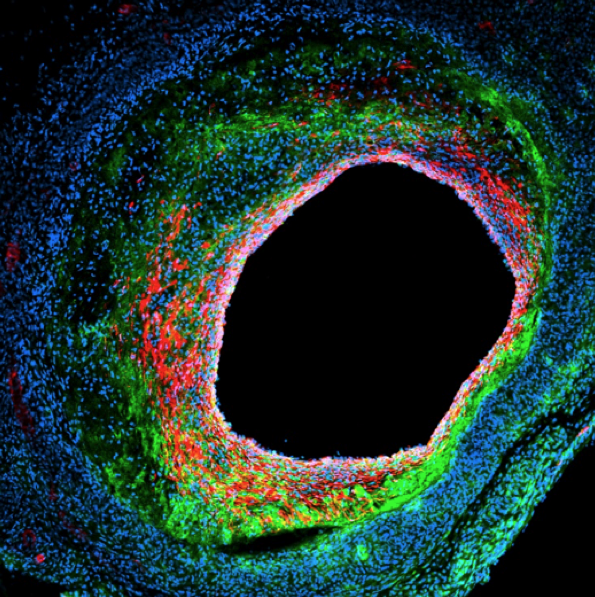
Mid cross-section of a mouse vein graft with plaque. Immunofluorescence shows macrophage marker CD68 (green), vascular smooth muscle marker (red) and endothelial marker CD31 (pink to white). DAPI nuclei staining are seen in blue.
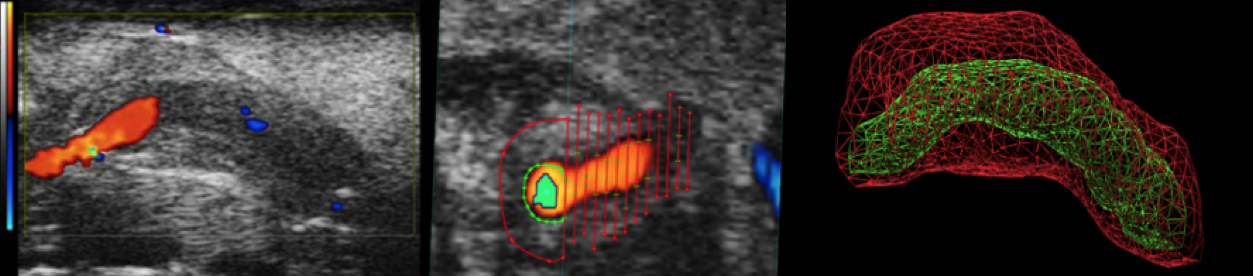
High resolution ultrasound imaging (Long axis view: Color Doppler-B Mode overlay) of a mouse vein graft at 4 weeks post implantation (left panel), its corresponding 3D imaging (Color Doppler and B Mode) showing stacked serial slices with plaque area measurements (middle panel), and 3D volume rendering showing outer vascular wall border in red and luminal border in green wire frames (right panel)
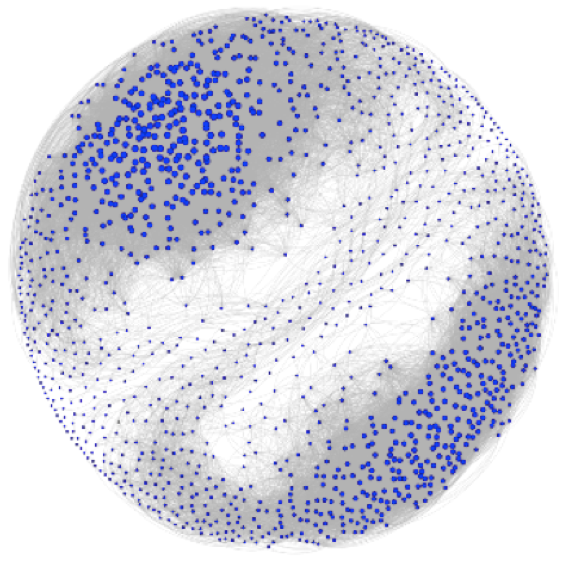
The co-abundance networks of M0, M1 and M2 macrophages, derived from proteomics measurements
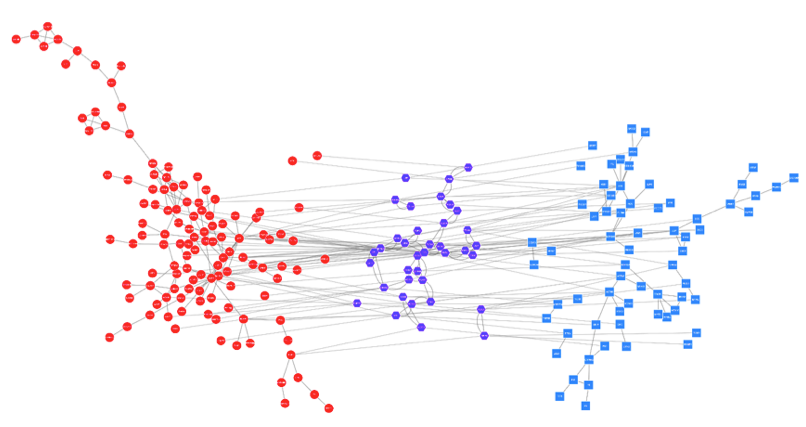
The merged network of cardiovascular related drug targets (blue squares), and disease genes (red circles).
About Us
Masanori Aikawa MD, PhD leads a diverse group of scientists, each driving a unique project with a focus on macrophage functionality in various cardiovascular-related diseases. In each of these diseases, macrophages are proposed to take a pivotal role in determining the disease state and pathology. Particularly, we are exploring the way(s) that macrophage ‘programming’ (the way they are driven to function within a particular disease state) impacts the progression and/or severity of a disease and, in turn, present a treatment target. Specifically, we are investigating the role of monocyte/macrophage activation in diabetes and the contribution of macrophage function in pre-clinical studies involving atherosclerosis, vein graft disease and abdominal aneurysm.
Vein graft bypass is a common surgical procedure to manage advanced stages of peripheral arterial disease that leads to critical limb ischemia. However, roughly 40% of vein grafts in the clinics fail within the first year of implantation. Our group is interested in elucidating the mechanisms of vein graft failure by looking into the roles macrophages play in the plaque formation and vascular wall remodeling in vein graft disease.
Using a mouse model of vein graft disease and abdominal aortic aneurysm, we aim to search for druggable targets, biomarkers of disease progression and future outcome, as well as novel mechanistic insights into vein graft disease, and aortic aneurysmal development.
Macrophage polarization is a highly complex process involving multiple factors. One particular area of interest includes investigating the role of PARP9 and PARP14, poly(ADP-ribose) polymerase family members, which we identified by global proteomics. We believe the interaction between the PARPs and STAT1/STAT6 signaling participates importantly in the polarization of macrophages. In another project, we are examining the role of non-coding RNAs in the regulation of macrophage biology.
With a multitude of trans-omic approaches, our group also uses network paradigm as a tool for understanding the systemic properties of biological systems. One aspect focuses on fusing proteomics data with network science tools to develop new techniques for therapeutic target prioritization and discovery, with the specific aim of understanding the inflammatory component in cardiovascular diseases.